Hemoglobin S (HbS) is an abnormal hemoglobin resulting from a single nucleotide substitution in the beta-globin gene. Individuals who are heterozygous for the HbS genotype are regarded as carriers of sickle cell anemia (SCA; HbSS) and are said to have sickle cell trait (SCT; HbAS). Recent data have established SCT as a risk factor for venous thromboembolism (VTE), chronic kidney disease (CKD), and end-stage renal disease (ESRD) (Naik RP, 2018). These complications have a relative risk of approximately 1.5 - 2.0 compared to race- and age-matched controls, but the mechanisms that account for these complications are not well understood.
To study potential mechanisms for renal disease, we evaluated red blood cell (RBC) deformability from SCT donors under conditions designed to mimic the hypertonic, acidemic, and hypoxemic environment of the inner renal medulla. RBC deformability was measured using the Laser Optical Rotational Red Cell Analyzer (LORRCA; RR Mechatronics, The Netherlands). In SCA, LORRCA has been used to monitor RBC rigidity at baseline and under progressive hypoxia by calculating the Elongation Index (EI; the longitudinal dimension of the RBC along the axis of applied shear stress divided by transverse dimension of the cell along the same axis). The OxygenScan function measures EI during a cycle of continuously decreasing pO2 from about 150 mmHg (ambient air) to near 20 mm Hg, before reversing in a re-oxygenation phase. In SS RBCs, EI decreases from a stable baseline as RBCs start to sickle; this ‘point of sickling (PoS)‘ is defined as the pO2 at which there is a >5% decrease in EI. Similarly, the Osmoscan function can record the RBC EI at ambient oxygenation while osmolality of the blood progressively increases from approximately 100 to 600 mOsm.
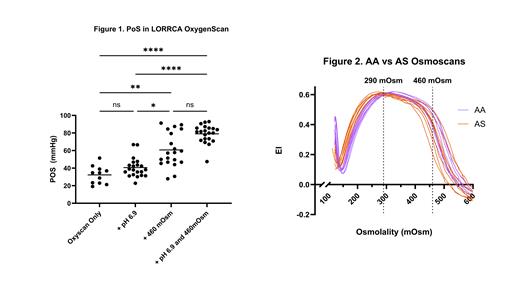
First, OxygenScan profiles were performed utilizing modified LORRCA buffers to evaluate four separate conditions: isotonic + neutral pH, isotonic + pH 6.9, 460 mOsm + neutral pH, and 460 mOsm + pH 6.9. Buffers were modified by the addition of sodium chloride, hydrochloric acid, or both. HbS percentage (HbS%) and RBC indices were recorded for each subject. HbS% and RBC indices were compared to PoS for all samples and conditions. EI from AS and AA samples were compared at physiologic serum osmolality (290 mOsm) and at 460 mOsm. AA samples demonstrated no measurable PoS under any condition. Some (11/21) AS samples demonstrated sickling under hypoxia alone, with mean PoS of 32.26 mmHg. Mean PoS for AS samples in isotonic buffer + pH 6.9 was 40.73 mmHg, but with sickling detected in all samples. Mean PoS in hypertonic buffer (460 mOsm) significantly increased to 60.68 mmHg, with notable inter-individual heterogeneity (Fig. 1). Mean PoS in hypertonic buffer + pH 6.9 was 79.04 mmHg, with less inter-sample heterogeneity. In exploratory experiments, we have observed similar changes in the Townes AS murine model of SCT (not shown). PoS was compared to HbS% in the human samples under all conditions. A positive correlation was observed between HbS% and PoS in isotonic buffer at pH 6.9 (r=0.566, p=0.012). PoS was also compared to RBC indices, with a trend towards a positive correlation with mean corpuscular hemoglobin concentration (MCHC) under hypertonic + neutral pH conditions ( p=0.11).
Next, utilizing the Osmoscan function, no difference in EI at physiologic osmolality (290 mOsm) was observed between AA (n= 7) vs. AS (n= 8). However, at 460 mOsm, EI trended lower in AS RBCs ( p=0.12) (Fig. 2).
Given the presence of severe tissue hypoxia (as low as 10 mmHg), hypertonicity (as high as 1,200 mOsm), and acidemia (as low as pH 5.5) in the inner renal medulla, these data provide an explanation for RBC-mediated occlusion of the vasa recta that is known to occur in the kidneys of subjects with SCT. We demonstrate that extracellular hypertonicity contributes significantly to SCT RBC sickling in the presence of hypoxemia, and that there is significant inter-individual susceptibility to sickling under these harsh biophysical conditions that is only partially explained by HbS%.
Disclosures
Ellsworth:Genentech: Consultancy, Honoraria; Novo Nordisk: Research Funding. Pawlinski:CSL: Consultancy, Research Funding. Key:Uniqure/CSL: Consultancy, Ended employment in the past 24 months, Other: HOPE-B trial (hemophilia gene therapy) Steering Committee; Genentech: Consultancy; Novo Nordisk: Consultancy, Other: Chair of hemophilia grants study section; Biomarin: Consultancy, Ended employment in the past 24 months, Honoraria.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal